Clinical Data Management: Process, Benefits, and Software Solutions
03 Jan 2024
18 Min
340 Views
Effective clinical data management is critical in modern healthcare to make better-informed decisions about diagnosis, treatment plans, and potential health risks. Healthcare organizations must meet the highest standards in data collection, processing, and storage, especially in clinical trials.
The importance of effective clinical data control manifests itself in improving the accuracy of diagnoses, ensuring patient safety, meeting regulatory compliance, and supporting research to enhance medical practice further.
In this article, we will explore the essence of the clinical data management process and its regulatory landscape, what software is used for it, and how to integrate the clinical data management system. You will also unveil why custom clinical data management software is better decision than ready-made one.
What Is Clinical Data Management and Why It’s Important
Clinical Data Management (CDM) is an integral component of clinical research operations allowing to manage all aspects of clinical trials including collecting, validating, storing, and managing data obtained during clinical trials. Clinical trials are scientific studies to assess the safety, efficacy, and quality of new medical treatments, drugs, or devices. Role of clinical data management is ensuring that the information generated from these trials is accurate, reliable, and adheres to regulatory standards.
Advantages of effective CDM
Clinical trial data management provides healthcare organizations with many advantages, including:
Data accuracy and integrity
Efficient document management in clinical trials minimizes errors in data entry, ensuring the precision and dependability of clinical trial information. Rigorous validation processes identify and rectify inconsistencies, upholding data integrity.
Compliance with regulatory standards
Adherence to health standards, including FDA (Food and Drug Administration) and ICH(International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) is paramount. Robust electronic clinical trial management solutions establish a compliant environment with audit trails that meticulously document data changes for regulatory scrutiny.
Timely and efficient data collection
Streamlined processes in clinical trials data management reduce the time and resources required for data entry and validation. Real-time monitoring enables swift issue identification and resolution, enhancing overall clinical trial study efficiency.
Patient safety
Effective clinical data management software facilitates early detection of adverse events, enabling prompt intervention to safeguard the well-being of study participants. Actionable insights support accurate assessments of treatment safety and efficacy.
Cost-efficiency
Data management in clinical research contributes to cost efficiency by automating and optimizing all aspects of clinical financial management processes. Fewer data queries and streamlined clinical operations minimize the need for additional resources, promoting resource optimization.
Data security and confidentiality
Integraton robust security measures in clinical data management solutions allows to protect sensitive patient information, ensuring compliance with privacy regulations. Access to study based on roles and permissions enhance data confidentiality in clinical applications.
Facilitated data analysis and reporting
Well-managed data sets form the basis for accurate statistical analysis, supporting meaningful interpretation of trial results. By merging and structuring data, clinical trial management system lead to faster generation of study reports and submission of regulatory documents.
Effective data review in clinical data management is pivotal for the success of clinical trials, providing a foundation of reliable, high-quality information essential for informed decision-making in healthcare and advancing medical knowledge.
Clinical Data Management Process Step-By-Step
Understanding the clinical data management process flow chart is critical to successful clinical research. This familiarity with each step of CDM helps ensure data accuracy, reliability, and security, contributing to quality study outcomes.
Let's review each clinical research data management stage.
Data Management Plan (DMP)
The Data Management Plan (DMP) is a detailed document that outlines all procedures, tasks, milestones, and deliverables throughout the data management clinical trial lifecycle. This document provides a roadmap for handling information and managing potential risks. It also serves the vital function of clearly communicating what is happening in the study to everyone involved.
The DMP typically describes the following aspects:
- Data collection. Defining the information gathered from participants
- Data integration. Incorporating existing data into the process
- Data format. Establishing the format for collected data
- Metadata and standards. Determining metadata and its standards
- Storage and backup. Outlining methods for data storage and backup
- Security measures. Implementing measures to safeguard sensitive information
- Data quality procedures. Ensuring the quality of collected data
- Team responsibilities. Assigning roles and responsibilities within the team
- Access and sharing mechanisms. Defining mechanisms and restrictions for data sharing
- Long-term archiving. Detailing procedures for long-term data archiving
- Cost considerations. Evaluating the expenses related to data preparation and archiving
- Regulatory compliance. Ensuring adherence to healthcare industry regulations and standards (e.g., HIPAA, GDPR, FHIR, HL7 integration, etc.)
The DMP should be ready during the study management platform design phase before enrolling the first participant.
Design of the electronic case report form (eCRF)
The electronic case report form (eCRF) is a questionnaire for collecting data from study participants and submitting it to trial sponsors. This document is explicitly created for each research project by the study start-up protocol and Clinical Data Standardization Consortium (CDISC) guidelines to unify data sharing across the industry.
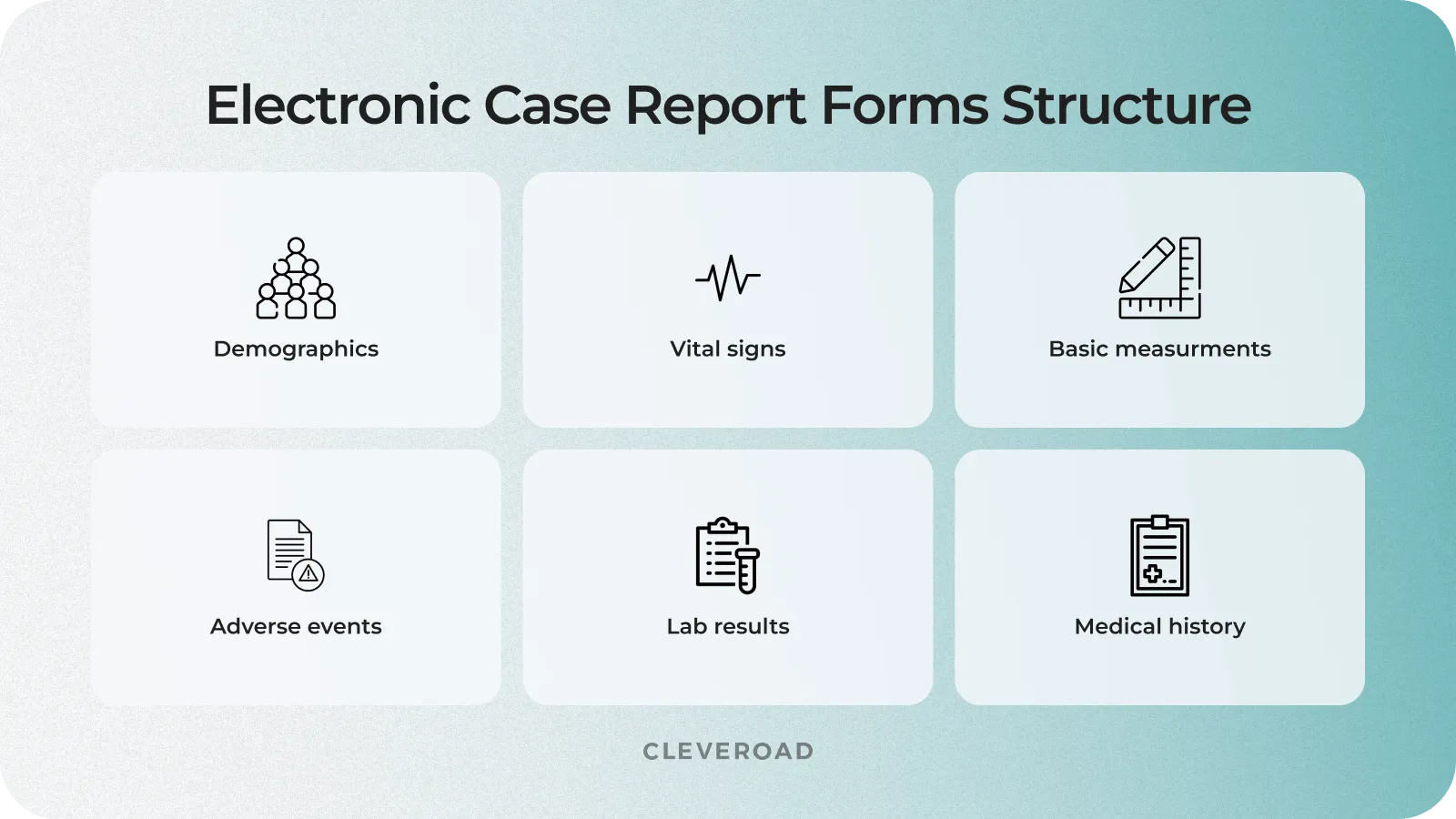
eCRF structure in clinical trial data management
Clinical trial database design
A clinical trial database is data gathered during a study and organized into rows and columns. It is designed with the structure of eCRFs kept in mind. Some questionnaire data may be coded into meaningful categories to save space in the database. In this case, database specialists create detailed decoding descriptions or techniques for mapping codes into CRF elements and standardize clinical data across trials. Before launching into the research environment, the database is tested with fictitious data in a secure setting.
Electronic data capture in clinical research
As mentioned earlier, CRFs (electronic or paper data collection forms) are the primary tool for obtaining information in clinical trials. Traditionally, clinicians or data entry specialists collected information for these forms from participants during their visits to healthcare facilities. However, current trends show that healthcare organizations are no longer the only research site. Details for content management are now also being extracted from various healthcare software and tools like Electronic Health Records (EHRs), medical devices, wearables, laboratory research management systems, etc.
Data validation
The validation phase of data management for clinical trials involves performing a series of tests to ensure the accuracy, consistency, readability, and integrity of data from different sources. This process includes such activities:
- Electronic checks. The database designer creates and embeds electronic checks into electronic CRFs to automatically compare input data against numeric and logical criteria, preventing unrealistic values from appearing in the document.
- Source Data Validation (SDV). This process involves verifying CRF data against original medical records and other sources to confirm that electronic CRFs contain all necessary information and faithfully represent the participant's profile.
- Data anonymization. Before submission, clinical data undergoes an anonymization process as required by law, removing all elements of protected health information (PHI) linking the document to a specific individual.
Database closure and data archiving
After the study, the database is locked to prevent the information from being altered. The clean data is then available to stakeholders for statistical analysis, reporting, and publication of results. However, all these steps are beyond the scope of clinical data management.
The necessary documents and study materials should be archived for at least three years, ensuring that the data can be monitored, evaluated, and recovered for future studies.
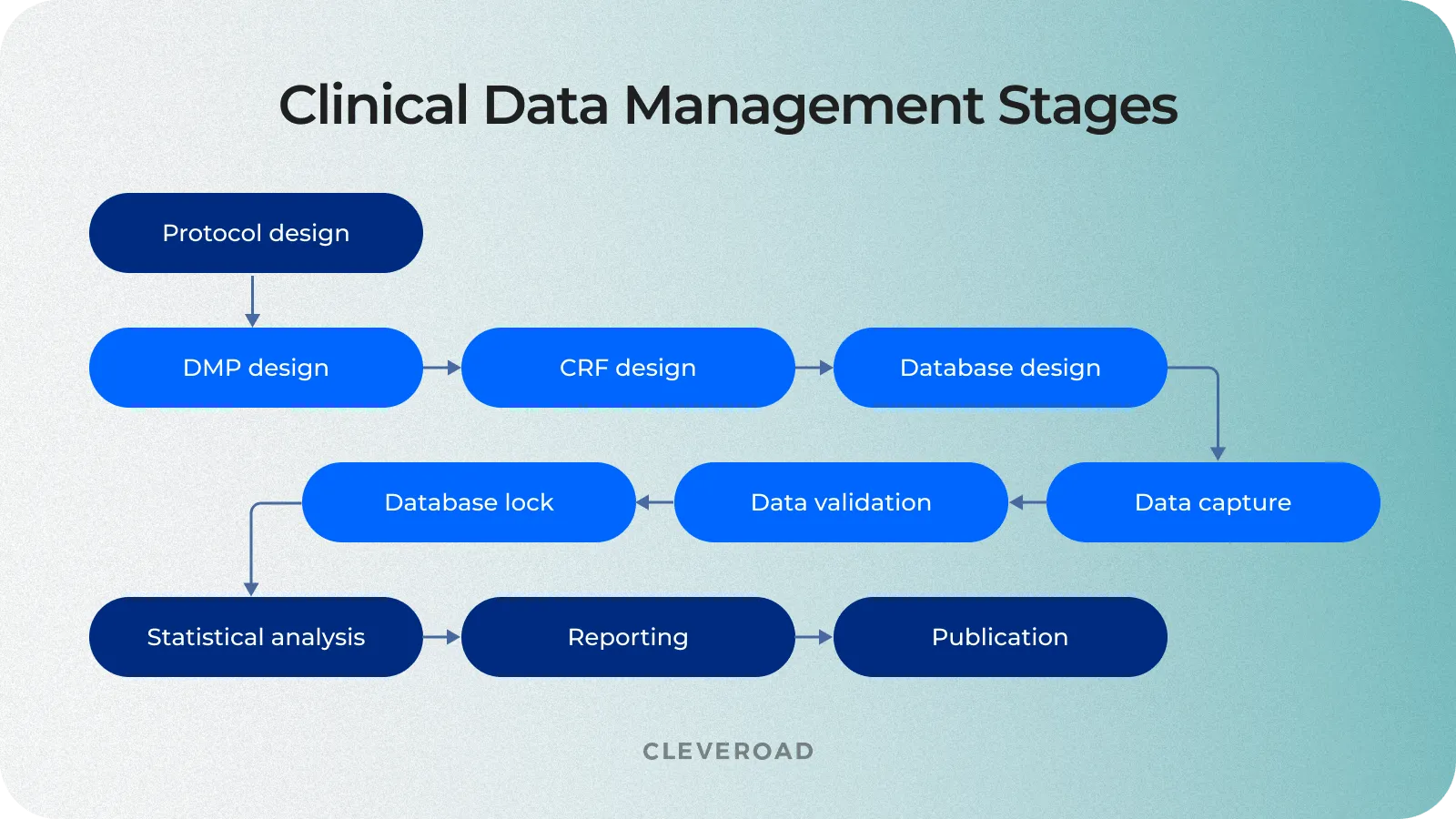
The process of clinical trial data management
Clinical Data Management Roles and Responsibilities
A well-coordinated team is crucial for smooth clinical trial operations. From project management to meticulous data entry and coding, each role ensures accurate and compliant data throughout the trial. This collaborative effort guarantees effective clinical suite data management, fostering transparency and precision in line with regulatory standards. The table below outlines the clinical data management roles and responsibilities.
| Role | Responsibilities |
Clinical data manager |
|
Database programmer/designer |
|
Data entry associates |
|
Medical coder |
|
Quality control associate |
|
The Most Successful Clinical Data Management Solutions
There is a large variety of software for clinical data management on the market, offering diverse tools. Let's take a look at some of the best clinical trial management software (CTMS) to explore their functionality and gain useful insights for creating your own solution:
IBM Clinical Development
IBM Clinical Development (ICD) is a leading clinical trial management cloud-based system designed for data capturing in large-scale, decentralized studies. This CTMS provides reliability, a rich library of pre-built forms, and a modular structure, allowing users to pay for specific features. The AI power of IBM Watson supports medical coding. However, users note drawbacks such as an archaic UI, slow customer support, and a higher cost of ownership, making it less accessible for startup projects.
Oracle Clinical
Oracle Clinical is a modern clinical trial solution distinguished by its extensive usage, providing reliability and adaptability to various clinical trials. With a customized pricing model, it offers rich functionality and seamless integration with the Oracle Health Sciences ecosystem. However, users highlight a steeper learning curve and potential costs associated with dedicated training.
Castor EDC
Castor EDC is famous for its ease of use and adaptability, serving over 2000 clinical trials. Users are choosing a CTMS platform for its transparent subscription-based pricing ensures clarity and features a user-friendly interface with high customizability. Automated data validation enhances accuracy, but users note limitations in advanced features for complex trials.
Medidata Rave
Medidata Rave is a widely adopted cloud-based clinical trial platform for over 3500 clinical trials. With flexible pricing based on study characteristics, it offers cloud accessibility to manage your clinical data anywhere, risk-based monitoring, and eSource capabilities. However, users are cautious about higher initial costs and potential ongoing expenses, requiring careful budget management.
OpenClinica
OpenClinica, utilized in over 1500 clinical trials, stands out for its open-source nature, adhering to CDISC standards. Its open-source flexibility and role-based access enhance customization and manage clinical data securely. However, users mention limitations in advanced features and a reliance on community support, particularly for complex clinical studies.
| Clinical Data Management Solutions | Used by Trials | Pricing Model | Pros | Cons |
IBM Clinical Development | 3000+ | Individual plans | Reliability, Coding with Watson AI, Extensive Library of Forms, Modular Structure | Archaic UI, High Price, Requires Programmer for Setup |
Oracle Clinical | 2500+ | Customized plans | Robust Feature Set, Comprehensive Data Management, Seamless Integration | Steeper Learning Curve, Potential Higher Costs |
Castor EDC | 2000+ | Subscription-based | User-friendly Interface, Customizable, Automated Data Validation | Limited Advanced Features, Not Ideal for Complex Trials |
Medidata Rave | 3500+ | Flexible pricing | Cloud-Based, Risk-Based Monitoring, eSource Capabilities | Higher Initial Costs, Potential Ongoing Expenses |
OpenClinica | 1500+ | Open source | Open-Source Flexibility, CDISC Standards Compliance, Role-Based Access | Limited Advanced Features, Reliance on Community Support |
Off-the-Shelf Vs. Custom CDMS
When deciding to build data management systems for clinical trials, you will be faced with a choice of two options:
- Use off-the-shelf CDM software
- Create custom clinical data management solution
Let's explore the features of each of these development approaches so that you can make an informed decision:
Off-the-shelf software
Off-the-shelf clinical trial software provides pre-packaged solutions for purchase and use. These solutions come with standard features and configurations for medical data administration, aiming to suit a broad range of users without extensive customization.
Pros:
- Quick enrollment. Off-the-shelf solutions can be deployed quicker, saving time on development.
- Lower initial costs. These solutions come with lower upfront costs than custom alternatives.
Cons:
- Limited customization. Lack of flexibility and customization options may lead to constraints in adapting to unique study requirements.
- Charges for unused functionality. You may have to pay for features that you don’t need, which, in the end, will be more expensive in the long run.
- Limited scalability. Even with top clinical trial management software, you can face challenges as the scale or complexity of the study increases.
- Generic user experience. Users may encounter a generic interface that does not cater specifically to their workflow.
Custom clinical data management software
Custom clinical data management software is tailored to the specific needs of your research organization or study. It involves developing a solution from scratch, ensuring that every aspect aligns with the study's unique protocols, workflows, and data formats.
Pros:
- Tailored adaptability. Custom solutions can be adapted precisely to the unique protocols and workflows of the study, offering maximum flexibility.
- Scalability. Designed to scale seamlessly with evolving project needs, minimizing disruptions and accommodating growing data requirements.
- Enhanced security. Custom CTMS software provides robust safety measures to secure data from research systems.
- Optimized user experience. Interfaces can be designed to maximize user experience, improving overall usability.
Cons:
- Longer development time. Building a custom solution may take longer due to the development process, testing, and fine-tuning. But such software will be tailored to your specific healthcare business needs, allowing you to boost clinical trial efficiency.
- Higher initial investment. The initial costs of developing a custom solution may be increased compared to off-the-shelf options. However, this can lead to potential long-term savings and efficiency gains.
In summary, while off-the-shelf software offers quick implementation and lower initial costs, the custom clinical data management solution provides tailored adaptability, scalability, security, and user experience, making it a preferred choice for organizations with specific and evolving needs in the clinical research domain.
| Aspect | Off-the-Shelf CDM Software | Custom CDM Software |
Implementation time | Quick implementation | Longer development and implementation time due to solution iniqueness |
Adaptability | Limited customization | Tailored to specific needs and workflows |
Cost | Generally lower upfront costs | Higher initial investment, potential long-term savings |
Scalability | Limited scalability | Adaptable and scalable as per evolving needs |
Security | Limitations in implementing security measures | Solid security measures based on requirements |
User experience | Generic interface | Tailored for optimal user experience |
Integration | Rescticted integration capabilities | Seamless integration with existing systems |
Regulatory Landscape in Clinical Data Management
The regulatory framework for clinical data management is shaped by various industry standards, laws, and regulations designed to ensure ethical behavior, data integrity, and the rights of clinical trial participants. Compliance with these regulations is critical to confirm the validity of trial results and the well-being of participants.
Here is an example of healthcare laws and standards to comply with:
Good Clinical Practice (GCP). GCP is the global standard for the ethical and scientific conduct of clinical trials. With several standard requirements for GCP-compliant data management, it ensures participants' safety and the trial results' reliability through rigorous standards.
Health Insurance Portability and Accountability Act (HIPAA). HIPAA ensures the privacy and security of individually identifiable health information in the United States. The regulation prescribes measures to protect the confidentiality and integrity of participants' medical data.
Read our guide to HIPPAA-comliant software development with no mistakes
General Data Protection Regulation (GDPR). GDPR in the EU regulates the processing and protection of personal data. Particular attention is paid to consent, data anonymization, and the right to erasure, which affects data from clinical trials involving EU residents.
Clinical Data Interchange Standards Consortium (CDISC). CDISC sets global standards for the structure of clinical trial data. The main point of the regulation is to promote data standardization to facilitate exchange, reduce ambiguity, and simplify review by regulatory authorities.
International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH promotes cooperation between regulatory authorities and the pharmaceutical industry. The primary purpose of harmonization is to interpret technical guidelines for drug registration.
21 CFR Part 11 - Electronic Records; Electronic Signatures. Part 11 of the US CFR establishes criteria for electronic records and signatures. It regulates electronic systems to ensure data integrity, security, and confidentiality in clinical trials.
Adhering to these healthcare laws and regulations is vital for clinical data managers, sponsors, and investigators. Compliance with these industry regulations validates trial results and advances medical knowledge and patient data safety.
Best Ways of Integrating a Robust Clinical Trial Data Management System
Now, let's consider the main points when designing and implementing clinical data management software.
Defining your healthcare business needs
Before creating a robust clinical trial data management system you must clearly define trial objectives, including identifying specific data, key performance indicators (KPIs), desired outcomes and other critical metrics. Understanding the trial's purpose is crucial for aligning data management efforts with overarching research goals. Also, early engagement with a study team and stakeholders, including researchers, clinicians, and data managers, ensures a comprehensive understanding of user needs.
Finding an experienced tech vendor
Selecting an experienced IT vendor providing quality healthcare software development services is a critical step. This includes researching the vendor's reputation, including checking reviews on platforms like Clutch and GoodFirms, reviewing previous projects, and evaluating their experience in creating solutions for healthcare and telemedicine. You should also ensure the vendor can build a custom clinical data management system that meets the unique needs of your healthcare organization.
You can review our Clutch profile to explore how we’ve helped our clients to solve their business challenges and their opinions about cooperation with Cleveroad.
Learn how to outsource healthcare software development from our detailed guide
Regulatory compliance and security
Compliance with industry and security standards is integral to implementing a clinical data management system. Data protection, privacy, and regulatory conformity are critical aspects of the process. Make sure your vendor is experienced in healthcare data security. For example, to ensure medical software safety, Cleveroad experts use security measures such as:
- Role-based access control and activity tracking
- Implementation of standards like HL7 and HTTPS/encrypted WebRTC
- Industry-standard data encryption at rest and on client devices
Ensuring adherence to healthcare laws and standards governing clinical trials is equally essential. This includes compliance with requirements for processing personal data (GDPR, HIPAA) and conformity with data storage and exchange standards, such as CDISC, HL7, and other relevant laws. Regulatory compliance ensures the legality and transparency of clinical trials, promotes trust among study participants, and supports ease of interaction with regulatory agencies.
With 11+ years of healthcare software development experience, our team will help you create clinical data management software in compliance with healthcare laws such as HIPAA, GDPR, CDISC, ICH, etc.
Technology stack selection
Identifying the right technologies is critical in implementing a clinical data management system. This includes deciding between cloud-based and on-premise options and assessing the selected technology's readiness for future innovations in clinical research. An experienced tech vendor specializing in creating medical software solutions will help you choose the best technologies to build reliable software for clinical data management for effective collection and recording of medical info.
Creation of a flexible system architecture
Developing a system architecture that is easy to change and scalable plays a crucial role in ensuring effective data management throughout the clinical trial lifecycle. This flexibility allows for rapid implementation of necessary changes, scaling to accommodate increasing workloads, and successfully adapting to the diverse requirements that may arise during the study process.
Development and implementation of a quality control system
Establishing a quality management system that includes data validation and auditing mechanisms is essential in ensuring the information's accuracy and validity. This step ensures that the data meet high industry standards, promoting confidence in the study results and ensuring their reliability.
The Cleveroad team has experience in developing Quality Management System - the solution for medical device manufacturers, aimed to automate document flow and processes needed for the FDA and ISO certification of medical devices’ production.
Staff training
For a Clinical Data Management System (CDMS), staff training is key. It's about giving clinical and data management teams the skills to use the system well. This includes hands-on sessions on data entry, validation, and compliance. Regular refreshers keep everyone up-to-date with the latest standards, fostering a culture of accurate and efficient clinical data handling.
Ongoing support
A solid support setup is vital for a Clinical Data Management System. This means having a dedicated team for CDMS-related issues. Using a ticketing system helps prioritize and solve problems quickly. Regular health checks and clear communication channels prevent and address technical hiccups. Continuous training ensures staff can handle new features or updates, keeping the CDMS running smoothly and in line with industry standards.
System monitoring and optimization
Systematic, real-time performance monitoring and optimization allows you to identify and fix problems, ensuring smooth operation. Analyzing utilization data will enable you to identify trends and make necessary changes to improve system efficiency, ensuring optimal system performance. You software vendor can provide you with professional support and maintenance services to ensure data management clinical trials software works properly.
Improve Your Clinical Data Management with Cleveroad
Cleveroad is a healthcare software development company from the CEE region. For more than 11 years now, we have been helping clinics and medical establishments improve patient care and increase efficiency by building custom and robust healthcare and telecare software solutions. Our healthcare software development services include creating various medical systems, such as telemedicine and remote patient monitoring software development, EHR/EMR and clinic management systems, medical billing software, health data visualization solutions, E-prescribing software, healthcare CRM’s, etc.
By cooperating with Cleveroad, you will get such benefits:
- Custom healthcare and telemedicine software development services according to your clinics particular needs
- Experience developing healthcare software compliant with industry laws and regulations, such as, HIPAA, GDPR, PIPEDA, ICH, HITECH, HL7, FDA, etc.
- Deep expertise in building robust healthcare solutions with industry-standard data security tools (RBAC, activity tracking, industry standard data encryption, etc.)
- Experience in healthcare legacy software modernization by implementing the latest technologies, advanced functionality, and enhanced security measures
- Cooperation with ISO 9001:2015 and ISO/IEC 27001:2013 certified company, ensuring quality and information security
- Flexible cooperation models that suit your needs: Staff Augmentation, Dedicated Team, Time & Materials, etc.
- Signing NDA per your request to ensure confidentiality of your business data from the first contact
To show you our experience, we would like to present one of our customers' successful case studies - Clinic Management System with EMR module.
Our client, a rehab clinic from the USA, approached us to help them build a custom clinic management solution for optimizing appointment management and overall workflow. Our team created a clinic management system and accompanying apps according to the client's requirements, user roles, and healthcare specifics. We also provided reliable data migration on AWS cloud hosting - the provider that meets all the requirements for HIPAA-compliant systems.
The developed solution included the following functionality:
- Patient profile and EMR with billing and user management
- Real-time booking for consultation appointments
- Admin panel for system management, including the ability to create users, manage user access, manage online consultations, appointments, access to them, and schedules
- A robust accounting system to structure payment data and information about all healthcare services provided and paid
- E-prescriptions solution helps select pharmacies, securely transmit recipes to them, and incorporate insurers' data
- Web and mobile applications for patients to access all clinic services online
As a result, our client received a bespoke medical solution covering appointment booking, real-time scheduling, e-prescriptions, and other functionality needed for effective management of administrative and operational activities in healthcare establishments. The clinic's data was transferred to new secure storage, meeting the requirements of HIPAA regulation. Integrated functionality allows the clinic to manage all the processes, optimize workflow, and exclude schedule conflicts' risk.
Build clinical data management software with domain experts
Our team with 11+ years of experience in Healthcare software development is ready to create a robust healthcare system to simplify the storage, extraction, and management of clinical data
A Clinical Data Manager plays a pivotal role in clinical research, overseeing the end-to-end process of collecting, validating, and managing data derived from clinical trials. This involves designing and implementing data collection systems, ensuring data accuracy and integrity, and adhering to stringent regulatory standards. The Clinical Data Manager collaborates with cross-functional teams, including researchers, statisticians, and IT professionals, to guarantee the reliability of clinical trial data.
Clinical Data Management (CDM) is a comprehensive discipline within clinical research that focuses on the systematic organization, validation, and secure management of data collected during clinical trials. CDM encompasses several processes, including developing data collection instruments, data entry, verification, coding, and implementing measures to ensure data security and regulatory compliance. The ultimate goal is to generate high-quality, accurate data supporting evidence-based healthcare decision-making.
The Clinical Data Management process is a well-defined series of steps that begins with creating a detailed Data Management Plan (DMP). This plan outlines various stages' procedures, milestones, and deliverables, including data collection, integration, format determination, security measures, quality assurance, team responsibilities, and regulatory compliance. The process extends through data validation, analysis, and data preparation for reporting, contributing to clinical trials' overall success and validity.
Managing clinical data involves a systematic approach that encompasses several key components. It begins with the design of electronic case report forms (eCRFs) tailored to the unique requirements of each clinical trial. Data collection is then conducted, often electronically, with embedded electronic checks to ensure accuracy. Source Data Validation (SDV) verifies data against original records, and data anonymization is performed to remove personally identifiable information. A secure database stores the information, and stringent access controls are implemented to protect sensitive data.
The initial step in Clinical Data Management is the development of a comprehensive Data Management Plan (DMP). This document serves as a roadmap for the entire data management lifecycle, outlining critical aspects such as data collection methods, integration processes, data format, metadata standards, security measures, team responsibilities, access mechanisms, long-term archiving procedures, cost considerations, and regulatory compliance strategies. The DMP is a foundational document created during the study design phase.
Clinical Data Management is paramount in clinical trials due to its multifaceted contributions. It ensures the accuracy and integrity of trial data, which is critical for drawing valid conclusions and making informed decisions in healthcare. CDM plays a pivotal role in compliance with regulatory standards, including Good Clinical Practice (GCP) and the Health Insurance Portability and Accountability Act (HIPAA), safeguarding participant rights and data confidentiality. Moreover, efficient data management streamlines processes, enhances patient safety by enabling prompt detection of adverse events and contributes to the overall success and efficiency of clinical trials.

Evgeniy Altynpara is a CTO and member of the Forbes Councils’ community of tech professionals. He is an expert in software development and technological entrepreneurship and has 10+years of experience in digital transformation consulting in Healthcare, FinTech, Supply Chain and Logistics
Give us your impressions about this article
Give us your impressions about this article