The Role of Clinical Trial Management Software in Clinical Research
27 Dec 2023
16 Min
366 Views
The healthcare clinical trials are in demand among patients. It is proven by statistics: Classic Clinical Trials states that there are 476,816 registered clinical studies by the end of 2023, whereas this number was equal to 437,514 at the end of 2022 and 399,485 clinical researches in December, 2022. According to Statista’s data, TOP-3 studies from 2017 to 2022 are dedicated to researching oncology, infectious diseases, and neurology treatment. These are serious health problems, and accuracy plays a pivotal role in their research, as well as the right medication prescription.
A robust and comprehensive clinical trial management software will help the researchers study the diseases more precisely. As a skilled healthcare IT service provider, we’ve systematized all you should know about creating clinical trial management solutions: the software’s role in research, business challenges, and their solutions, as well as solution development steps.
What Is Clinical Trial and What Software Is Used?
Before starting to use the medicament or a treatment device, the biotech, pharma or medical device manufacturer launches the clinical trials – tests intended to verify the safety and effectiveness of the newly created medications – and invests in them. The aim of trials is to get an accreditation from a healthcare regulatory body, like Food and Drug Administration (FDA). That’s why, the company often applies to a Contract Research Organization (CRO) that starts research on sponsor’s behalf.
Let’s discuss the essentials about clinical trial research operations for you to dive deeper.
- Before the clinical trial starts, two electronic documents are created that will be essential for further research: the Case Report Forms (CRF) and the protocols to outline the plan and guidelines for the study. Moreover, physicians ask for approval from ethics committees and regulatory authorities before recruiting participants. It’s required to meet ethical standards and comply with regulations. Once approved, physicians find eligible patients, explain trial details, and get informed consent.
- At the start of the clinical trials, a baseline assessment is conducted for each participant, capturing their current health status and establishing a reference point for future evaluations. This includes a comprehensive medical examination, laboratory tests, and baseline assessments specific to the study's objectives. These initial measurements provide crucial insights into the participants' health conditions before the implementation of the experimental intervention, serving as a vital baseline for comparison throughout the trial.
- During the clinical research, the personnel also need electronic Patient Reported Outcomes (ePRO) systems. ePROs involve the use of digital tools or devices to collect data (e.g., illness symptoms, experiences, and overall well-being, providing real-time actionable insights into the impact of the treatment or intervention) directly from the patients participating in the trial.
- After a trial, the collected data is thoroughly analyzed with the help of various clinical trial software to conclude the safety and effectiveness of the tested intervention. This analysis involves statistical methods to determine if the results are statistically significant. The trial results, documented in the report, contribute valuable information to medical knowledge, helping researchers and healthcare professionals make informed decisions about new treatments and interventions.
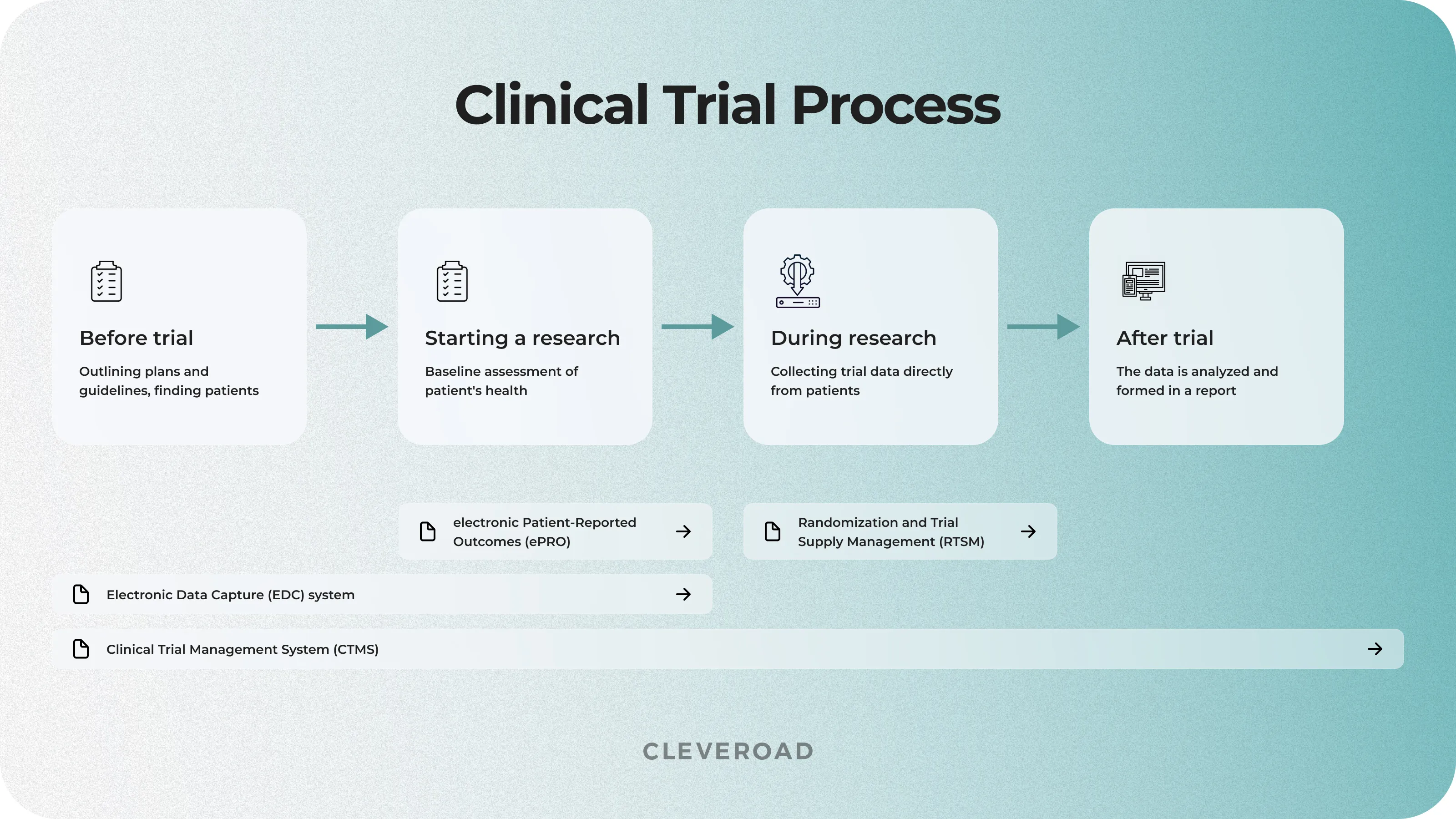
Clinical trial process and electronic documents engaged
The research workflow is quite complex, so the personnel use different healthcare software solutions that simplify the medical trial process and boost its effectiveness.
What digital solutions are used for medical trial
Let’s talk more about the software for clinical trials, and how researchers utilize it during the whole process of medical research.
Electronic Data Capture (EDC) systems are specialized software solutions that collect, manage, and store clinical trial information electronically. The essence of EDC lies in replacing traditional paper-based data collection clinical operations, offering a more efficient and accurate means of gathering and monitoring information in real-time during clinical trials. This medical research software is crucial to enhance the data quality and speed of collecting data from different sources, ensuring that clinical trial results are reliable, consistent, and compliant with regulatory standards. Furthermore, this kind of health trial software often includes features such as data validation checks, audit trails, and secure data storage to maintain the integrity of eclinical data for trials.
Electronic Patient-Reported Outcomes (ePRO) are digital tools intended to collect data directly from patients participating in clinical trials. It means the patient-reported information about their symptoms, experiences, and overall well-being can be collected in real-time and done accurately. The ePROs are successfully used to capture subjective data directly from the individuals undergoing the treatment, providing a more accurate and comprehensive understanding of the treatment's impact on their lives.
Randomization and Trial Supply Management (RTSM) software ensures that trial participants are assigned to treatment groups in a controlled and unbiased manner. This health system also helps physicians optimize the allocation of investigational products, manage inventory, and maintain the blinding of the trial. Moreover, the RTSM users can enhance the reliability and integrity of the trial results, reducing the potential for bias in participant assignment and ensuring the efficient management of clinical trial supplies.
Clinical Trial Management System (CTMS) is a centralized platform for medical researchers and trial coordinators to oversee various aspects of the clinical study, including patient recruitment, budgeting, regulatory compliance, and monitoring of milestones. The CTMS software improves the efficiency and organization of clinical trial processes, contributing to the successful trial execution.
Challenges of Clinical Trials and How CTMS Platforms Can Help
Now, we’ll discuss the types of clinical major challenges during the trials and how the specialized software can help solve them.
Clinical researches complexity
Hospital trials have been becoming more complex over the years. Clinicians conducting them are under pressure to create researches with accurate and comprehensive results for patients while also meeting the standards of regulators and payers. Clinical research sponsors must leverage the right technology and processes to simplify their research.
Clinical trial management system is a perfect way to achieve this goal. It facilitates efficient data collection, reducing the reliance on manual methods and minimizing errors. Additionally, such a health solution often integrates with Electronic Health Records (EHRs) and other data sources, fostering seamless data flow and enhancing overall trial efficiency. Clinical trial software enhances collaboration among research teams, allowing real-time communication and document sharing.
Regulatory compliance
Clinicians often feel restricted by the complexity of the healthcare guidelines they follow due to the heavily regulated nature of their industry. Furthermore, there are consistent differences among various regulatory bodies and the obstacles they present. As clinical trials are expanding to emerging markets in new countries, it is important to gain an understanding of the new healthcare requirements.
Hospital trial software can help Clinical Research Organizations (CROs) and other healthcare facilities better handle the regulatory compliance requirements for clinical trials. Thus, it’ll provide tools to manage and monitor different regulations and guidelines from different regulatory bodies (e.g., CFR, GPR, ICH-GCP). In addition, clinical trials software can offer real-time updates on regulations and guidelines from multiple jurisdictions, ensuring that healthcare organizations can quickly respond to regulatory landscape changes.
How to comply with HIPAA while developing healthcare software? Our guide will reveal it!
Patient engagement
The recruitment and retention of patients can present challenges for healthcare trial organizers due to difficulties meeting enrollment targets and increasing drop-out rates. Furthermore, patient engagement during clinical trials researches an additional challenge, as maintaining participants' interest and commitment throughout the study duration can be hindered by complex trial protocols or a lack of user-friendly communication channels.
The custom clinical trial management software addresses this issue by fostering better communication, and providing user-friendly interfaces to enhance participant engagement. Moreover, the hospital research software can customize the patient experience, allowing them to receive tailored notifications, reminders, and educational materials based on their preferences. It can also provide researchers with insights through data analytics and reporting tools given by clinical suite of digital tools to better understand their participants’ progress.
Engaging patients through health software: Hospital and lab content management system for patients developed by Cleveroad
Why Clinical Trial Management Software
The clinicians use different kinds of healthcare trial software like RTSM, ePRO or EDC depending on the specific trial activities and aims of their workflows. However, we’ll talk more about CTMS as the powerful instrument for streamlining and managing the entire trial process.
Let’s discuss how software for trial management benefits clinical researchers and healthcare providers and helps them manage their activities.
Getting accurate trial data
Accessing both general patient information and specific details can sometimes be challenging, such as obtaining an up-to-date list of websites or coordinating visits to startup companies. This method does not yield satisfactory results in conducting a clinical study. CTMS platforms provide transparency and centralized access to study information about a patient's health, allowing the study team to efficiently fulfill their responsibilities and make well-informed decisions.
Facilitating and supporting collaboration
Team members have the ability to work together on a single task for the same study management, like study startup, knowing that they are all accessing the most up-to-date data across various sources. Sponsors, CROs, Sites, and other vendors have the opportunity to collaborate and share the responsibility of maintaining accurate study tracking data.
Effective health studies planning, tracking, and monitoring
For instance, the CTMS provides an electronic visit report authoring feature that integrates visit details (e.g., study, site, investigator name or date) automatically. Your medical staff will also be provided with checking for completed sections, which cannot be reached through managing patient data with the help of Word. Another example of the effective CTMS functions performance is the Payment feature that generates site payment tracking records automatically based on contract information when subject visits are finished.
Clinical research transparency
The trial management software provides transparency in managing various aspects of your study, including study initiation, participant screening and enrollment, document management and collection, site visits, monitoring reports, completion of subject visits, handling action items and issues, and more. Admin dashboards and data reports offer visual representations and scoring of performance for individual studies or combined views of multiple studies.
Moreover, you can integrate CTMS software with different systems and databases to provide its multifunctionality. Based on our experience in building healthcare software, we advise to use the following tools for clinical trial study through CTMS integration:
- Regulatory repositories
- Interactive voice response systems
- EHR/EMR software development
- Electronic Data Capture (EDC) systems
- Safety databases
- Departmental databases
- Healthcare APIs
EHR software interface developed by Cleveroad
Best Clinical Trial Management Systems on the Market
Emphasizing an essential role of CTMS software in integrating the essential elements of clinical trials and research, we’ve gathered TOP-5 best management systems chosen by leading clinical trials companies for you to consider their value and feature set.
1. Main EDC
The Main EDC is a clinical trials management software that offers a variety of integrated tools, including EDC, IWRS, drug and supply management, and ePro. It also streamlines trials and studies conducted by clinical research organizations, Big Pharma, and biotech, regardless of their complexity, due to its artificial intelligence capabilities. Additionally, the solution studies management platform employs blockchain technology to ensure a comprehensive and precise record of your data, which is stored in certified data centers.
Its main functionality includes:
- Built-in analytics and reporting capabilities
- Interactive admin dashboards
- Visit&trial structure
- eCRF instrument for design approval
- AI-empowered healthcare coding
2. EDGE
EDGE software for clinical trials was developed at the University of Southampton's Clinical Informatics Research Unit. The purpose of this CTMS system is to provide researchers with a platform where they can efficiently track their studies and access research data in real-time. This is a collaboration platform that aims to prevent task duplication and streamline efforts, while also prioritizing the security and confidentiality of patient data.
There are the following EDGE core features this software has:
- Patient monitoring
- Tracking and reporting
- Appointment/event monitoring
- Shared calendars
- Finance management, recording and tracking
- Management of documents
3. Florence eBinders
Florence eBinders is a clinical trial solution that provides accessibility and security for investigator files, logs, and participant binders. You can customize your flows to fit your study and site needs. The CTMS software is used by trial sponsors, CROs, medical device companies, and research sites that conduct multiple studies in multiple locations.
It offers the following functionality:
- Remote monitoring options
- Interactive dashboard
- Compliant eSignatures
- Virtual workflows
- Task management
- EHR/EMR functionality
4. RealTime CTMS
RealTime CTMS clinical trials management software assists in managing research sites, sponsor data, and CROs. The software includes scheduling tools automating and streamlining study targets to send alerts for upcoming tasks. It also makes patient tracking easier with user-friendly features for data entry, enrollment, and data access. Moreover, this solution offers extensive reporting features for study metrics and staff productivity.
The system’s core features include:
- Financial management and tracking
- Task management
- Patient visit monitoring
- Analytics and reporting
- Mobile app referral capabilities
5. Sitero
Sitero provides a comprehensive management software for clinical trials in various therapeutic areas, including cardiac safety, infectious diseases, hepatology, neuroscience, respiratory, and oncology, among others. This CTMS software is designed to provide organizations with a comprehensive trial platform that is seamlessly integrated with an Electronic Trial Master File (eTMF), resulting in improved efficiency, accuracy, and security in consolidating clinical data.
Sitero’s core features include:
- Contact management
- Automated workflows
- Role-Based Access (RBAC) and views
- Inventory control module
- Payment module
The given CTMS platforms have demonstrated to you the useful core features you can integrate in your clinical trial management system to benefit from its transformative potential in research flows. Now, let’s cross the line from theory to practice and talk more about the CTMS development process.
Clinical Trial Software Development Steps
While planning your CTMS building, you can think out two development approaches:
- Off-the-shelf medical trial software creation
- Custom research system’s building.
Implementing off-the-shelf CTMS software is quite a fast way to achieve what you need for further trials. But be cautious: choosing a CTMS which is ready-made to use for your internal hospital research flows, you may fall into unforeseen expenditures connected to the features you won’t require.
In order to avoid it, you can turn to a clinical research system’s development from scratch. Below we look through the steps you should take during the process.
Step 1. Find a tech team to collaborate with
Working on CTMS software creation, you should start with ensuring the support of a skilled healthcare IT development team which is crucial for the success of your future product.
For instance, to find a tech partner fast, you can consider outsourcing healthcare software development to an experienced IT team for several reasons:
- You’ll access a specialized team that possesses domain-specific knowledge and experience
- You will communicate with the IT specialists understanding the intricacies and compliance requirements of the software development for clinical research industry
- You’ll get significant cost advantages
- You will tap into a global talent pool without the need for in-house resources.
When choosing a tech team, pay attention to their track record in developing healthcare and telehealth solutions. Look for teams that prioritize data security, are aware of regulatory compliance, and offer ongoing support and maintenance services to assure your CTMS stays robust and adaptable to industry changes. Client testimonials, case studies, and a transparent communication process are additional factors to consider in making an informed decision.
Cleveroad is capable of helping you with the CTMS building. We’ve got 11+ years of hands-on experience in healthcare software development. Moreover, we created medical systems for clinics, hospitals and medical facilities to effectively and securely manage patient data, minimize paperwork in medical facilities, as well as assist physicians with providing health analytics, and many more.
Step 2. Plan your activities with clinical trial IT specialists
To start collaboration with a tech team you’ve chosen, you should only contact them. For example, you can send us an application on Cleveroad website or write a software development RFP mentioning the details of your project. Our Healthcare Business Development manager will thoroughly study your represented information and contact you back to schedule a meeting for further project discussion and collecting more specifics about your CTMS concept.
Then, the tech team passes to the solution design stage, responding to your initial questions connected to clinical trial management systems development and establishing a meeting with the Solution Team (including business analysts, solution architect, and a UI/UX designer) to define your business needs and constraints to form a proposal and a cost estimate.
In order to turn the requirements into a clear CTMS creation plan, the team starts rendering the Discovery phase services. These services are aimed at developing your future CTMS architecture, detailed feature list, as well as building a design concept of how your future clinical research system will look. The healthcare solution architect also defines the medical regulations and standards the trial software should comply with like CDISC, FDA, HIPAA, 21 CFR Part 11: Electronic Records; Electronic Signatures, etc., as well as security measures. Finally, the specialists will represent you with a detailed cost estimate to clearly understand your future project expenditures.
Step 3. CTMS software development, QA and release
When the clinical trial software concept of interface is ready, the UI/UX designers hand their work over to developers. The primary aim of the programmers is to encode the pre-defined list of CTMS functionality for the trial solution created as one for the internal needs of your clinic or done as SaaS software development. The work is divided into several sprints (two-week periods to create a certain piece of functionality for a trial management software) and headed by project management specialists.
The QA engineers work together with developers, checking every created CTMS feature for bugs. If something is found, they send bug reports to the developers to make hotfixes. Moreover, when the feature list encoding is finished, they conduct the testing of your trial management software before the release. It is required to ensure the CTMS works flawlessly and without any possible constraints.
Our team will also help you with deployment of your CTMS platforms in your medical facility environment (in case you’re a healthcare facility, pharma company or a research institution). It can also be deployed as a SaaS software for different organizations and users depending on your business purposes. If you are satisfied with the outcome, we pass project materials (e.g., source code, specification, or design link) to you.
Consider more about custom systems creation with our healthcare software development services!
Step 4. Clinical trial system maintenance and support
The cooperation with the tech team doesn’t end after the CTMS release. As the clinical trial management is a complex process, requiring flawlessness, you may need support services for your newly created medical research software.
It means the specialists will constantly monitor your CTMS solution, checking it for bugs, upgrading on the basis of the collected early user feedback or adding new features. Considering the intricacies of the modern clinical software creation process, outsourcing your project to a healthcare IT development team can offer both experience and cost advantages.
Now, let us represent our company as the experienced healthcare software development vendor, and tell you more about our activity.
Cleveroad Expertise in Software Development for Healthcare and Telemedicine
Cleveroad is an experienced healthcare software development vendor with 11+ years of experience in designing and building the medical solutions for hospitals, clinics and other medical facilities around the world. Our R&D centers are located in Central and Eastern Europe (CEE region), notably Estonia.
You should collaborate with us because you’ll get:
- Custom healthcare software development. Obtain custom CTMS systems for clinical trials with seamless clinical data management that integrate smoothly into your existing workflows while addressing specific aspects of your business.
- Collaboration with an ISO-certified vendor. As an ISO-accredited software development provider according to certifications for ISO 27001:2013 (security) and ISO 9001:2015 (quality) Standards, we will help you create a qualitative and secure software for streamlining clinical trial processes.
- An IT team experienced in building legislation-compliant healthcare software for medical regulations and standards, such as HIPAA, PIPEDA, HITECH, HL7, GDPR, and more.
- A range of on-demand IT services offered including medical app development from scratch, UI/UX design, IT consulting, software modernization services, among others.
- Extensive experience in developing secure healthcare platforms for clinics, utilizing measures such as role-based access control, industry-standard data encryption, and activity tracking.
Feel free to check our recent healthcare software development cases in view of clinical applications from the portfolio.
- Quality Management System (QMS) development. Modernization of a B2B SaaS system to automate and streamline document flows and processes needed to ensure conformance with FDA and ISO requirements of medical device production.
- Clinical Management Software. A platform for the remote clinical data management for medical experts working in a telecare rehab and their patients. The system contains an EMR module and an easy-to-navigate mobile application to receive telecare services conveniently.
- RPM system for mental health support. This system designed for remote tracking of behavioral and overall health conditions to improve well-being of our client’s patients.
Work with an experienced medical IT partner
We’ll gather a team skilled enough to create a resilient clinical trial software – contact us and get full assistance
Clinical Trial Management System (CTMS) is a centralized platform and system of record for medical researchers and trial coordinators to oversee various aspects of the clinical study, including patient recruitment, budgeting, regulatory compliance, and monitoring of milestones.
The CTMS software improves the efficiency and organization of clinical trial processes, ultimately contributing to the successful execution of the conducted research. This technology enhances various aspects of clinical trials including the management of participant data, tracking of study progress, and facilitation of communication among research teams.
The essence of EDC lies in replacing traditional paper-based data collection methods, offering a more efficient and accurate means of gathering and monitoring information in real-time during clinical trials. CTMS in its turn, manages all aspects of clinical trial processes.
CTMS in clinical trials is a information management system that streamlines the research processes and helps the clinicians perform their duties legally and in compliance with the international healthcare regulations. The electronic clinical trial management system plays a crucial role in enhancing efficiency and ensuring adherence to regulatory standards throughout the trial lifecycle.

Evgeniy Altynpara is a CTO and member of the Forbes Councils’ community of tech professionals. He is an expert in software development and technological entrepreneurship and has 10+years of experience in digital transformation consulting in Healthcare, FinTech, Supply Chain and Logistics
Give us your impressions about this article
Give us your impressions about this article